| CHEMISTRY OF SODIUM HYDRO
CHEMISTRY OF SODIUM HYDROXIDE |
|
| Commercial name : caustic soda | |
| On industrial scale sodium hydroxide can be prepared by the following methods. 1) Castner – Kellner Process. 2) Gibb’s Method. 3) Nelson’s Method. |
|
| Castner – Kellener Process | |
| Principle | |
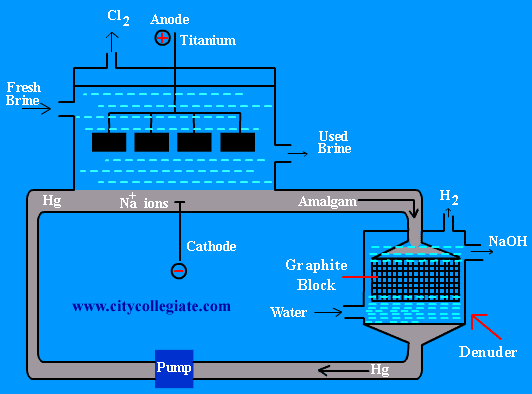
| In castner-kellner method NaOH is prepared by the electrolysis of aqueous solution of NaCl(Brine). | |
| Concentration of brine | |
| 25 % mass/mass i.e. 25 gm of NaCl is dissolved in 75 gm of water. |
|
| Castner-kellner cell | |
| It is a rectangular tank of steel. Inside of tank is lined with “ebonite”. Anode is made of titanium. Flowing layer of mercury (Hg) at the bottom of tank serves as cathode. |
|
 |
|
|
Details of process |
|
| Ionization of NaCl 2NaCl è 2Na+ + 2Cl– |
|
| When electric current is passed through brine, +ve and -ve ions migrate towards their respective electrodes. Na+ions are discharged at mercury cathode. The sodium deposited at mercury forms SODIUM AMALGAM. Chlorine produced at the anode is removed from the top of the cell.
|
|
| Reaction at cathode | |
| 2Na+ +2 e– è 2Na | |
| Na forms amalgam. | |
| Na + Hg è Na/Hg | |
| Na+ ions are discharged in preference to H+ ions due to high over voltage. Na+/Na: E.P. = -2.71 volt H+/H : E.P. = 0.00 volt |
|
| Reaction at anode | |
| 2Cl– è Cl2 + 2e– | |
| Formation of NaOH | |
| Amalgam moves to another chamber called “denuder”, where it is treated with water to produce NaOH which is in liquid state. Solid NaOH is obtained by the evaporation of this solution. | |
| 2Na/Hg + 2H2O è 2NaOH + H2 + 2Hg | |
| Advantages of castner’s process | |
| NaOH obtained is highly pure. The process is very efficient. Possible reaction between NaOH and Cl2 is avoided as NaOH is obtained in a separated chamber. |
|
| Disadvantages | |
| High electricity consumption. Environmental pollution due to escape of Hg vapours. |
|




