Direct anodic hydrochloric acid
Abstract
Hydrochloric acid (HCl) and caustic (NaOH) are among the most widely used chemicals by the water industry. Direct anodic electrochemical HCl production by water electrolysis has not been successful as current commercially available electrodes are prone to chlorine formation. This study presents an innovative technology simultaneously generating HCl and NaOH from NaCl using a Mn0.84Mo0.16O2.23 oxygen evolution electrode during water electrolysis. The results showed that protons could be anodically generated at a high Coulombic efficiency (i.e. ≥ 95%) with chlorine formation accounting for 3 ~ 5% of the charge supplied. HCl was anodically produced at moderate strengths at a CE of 65 ± 4% together with a CE of 89 ± 1% for cathodic caustic production. The reduction in CE for HCl generation was caused by proton cross-over from the anode to the middle compartment. Overall, this study showed the potential of simultaneous HCl and NaOH generation from NaCl and represents a major step forward for the water industry towards on-site production of HCl and NaOH. In this study, artificial brine was used as a source of sodium and chloride ions. In theory, artificial brine could be replaced by saline waste streams such as Reverse Osmosis Concentrate (ROC), turning ROC into a valuable resource
Methods
Electrochemical cell and operation
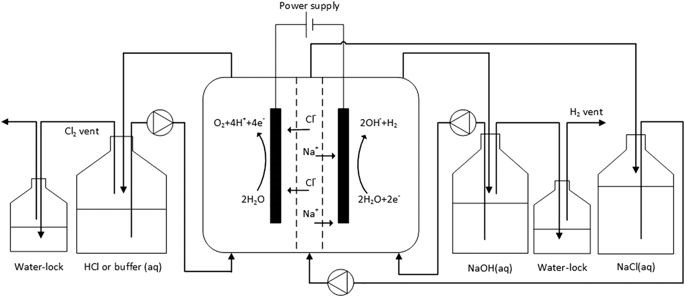
The methods used for the electrode preparation and characterization are described in detail in Supplementary Information9,18,19. Figure 1 presents a schematic overview of the experimental setup. The three-compartment electrochemical cell consisted of three Perspex frames with internal dimensions of 20 × 5 × 1 cm creating volumes of 100 mL for each compartment. An AEM (Ultrex AMI-7001, Membranes International Inc., USA) separated the anode from the middle compartment while the cathode and middle compartments were separated by a CEM (Ultrex CMI-7000, Membranes International Inc., USA). The produced mesh shaped Mn0.84Mo0.16O2.23 coated titanium electrode and a stainless steel mesh (6 mm mesh size, 0.8 mm wire) with a 24 cm2 projected surface were used as the anode and cathode material, respectively. All solutions (i.e. anode, cathode and middle solutions) were recirculated at a flow rate of 4 L/h using a peristaltic pump. An Ag/AgCl reference electrode (assumed at + 0.197 V versus NHE) was used in the anode compartment. Experiments were galvanostatically controlled at a current density of 250 A/m2 using a Wenking potentiostat/galvanostat (KP07, Bank Elektronik, Germany). Electrode potentials were recorded every 2 minutes using a data acquisition unit (Agilent Technologies, USA). Water-locks were used in the anode and cathode compartments to prevent oxygen (anode) and hydrogen gas (cathode) build-up. A 200 mL caustic solution (2 wt%) was used as the anode water-lock to trap any chlorine gas formed.
Figure 1: Schematic overview of the experimental setup.
Procedures of experimental runs
Preliminary results showed that the prepared Mn0.84Mo0.16O2.23 coated titanium electrode had a much lower affinity towards chlorine evolution than commercially available Ta/IrOx coated titanium electrodes (see Supplementary information). Subsequently, two sets of 4-hour experimental runs were conducted. The first set of experiments (n = 3) was conducted to determine the efficiency in terms of HCl and NaOH production. A 300 mL HCl solution (1 g/L) was used as the anolyte and a 300 mL NaOH (1 g/L) was used as the catholyte to provide sufficient initial conductivity. A 1L NaCl solution (35 g/L) was used in the middle compartment. At the end of each experiment, liquid samples from the anode and cathode were taken for measurements of HCl and NaOH, respectively. The second set of experimental runs (n = 3) was performed to confirm that chlorine formation was negligible. A 1 L solution of 40 g/L NaHCO3 and 1 g/L NaCl was used as the anolyte and a 500 mL NaOH solution (1 g/L) was used as catholyte. A 1L NaCl solution (35 g/L) was used in the middle compartment. The NaHCO3 solution was used to maintain its anodic pH level > 7.5, thus any formed molecular chlorine would remain in the solution as hypochlorous acid and hypochlorite ion rather than chlorine gas. As such, the CE for chlorine formation can be determined accurately. Liquid samples from the cathode were taken for measurements of NaOH production after 4-hour operation. At the end of each experiment, liquid samples from the anode were taken for measurement of the chloride and chlorine concentrations and the final pH values of all compartments were also measured.





Leave A Comment